
What is intrahepatic cholangiocarcinoma (iCCA)?
iCCA is a subset of cholangiocarcinoma (CCA)
CCA, the most common primary malignancy of the bile duct, is classified by anatomic origin into iCCA and extrahepatic CCA (eCCA). eCCA is further divided into 2 types:1-3
- Perihilar CCA (pCCA) (also termed “Klatskin’s tumour”) is the most common form, accounting for 50–70% of all CCA cases1,3
- Distal CCA (dCCA) accounts for 30–40% of total CCA cases1,3
iCCA originates in the bile ducts within the liver and accounts for <10% of CCA cases1,3
CCA is classified by anatomic origin3,4
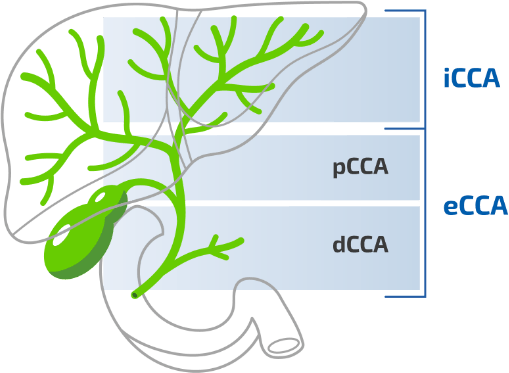
CCA, cholangiocarcinoma; dCCA, distal cholangiocarcinoma, eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma, pCCA, perihilar cholangiocarcinoma.
Figure adapted from Blechacz B. 2017.4
































